Belatacept is a selective T-cell co-stimulation blocker used in maintenance immunosuppression for kidney transplant recipients (KTRs), but evidence on cancer risk and other outcomes is limited. This retrospective cohort study used linked US transplant and cancer registry data on KTRs treated with belatacept (N=1514) or tacrolimus (N=7570) as initial maintenance therapy.
Transformar la vida de las personas con cáncer y trasplantes de órganos a través de la atención a la salud y la investigación integradas.

Ver vídeo de introducción (4 min)
El Centro de Innovaciones en Cáncer y Trasplantes se creó con los siguientes objetivos:
- Brindar una excelente atención clínica multidisciplinar a pacientes con cáncer antes y después de un trasplante.
- Liderar investigación excepcional, multidimensional y centrada en el paciente del cáncer antes y después del trasplante de órganos con un biorregistro extenso y una red de investigación colaborativa.
Buscarnos lograrlo con una clínica pionera, un biorregistro nuevo, y investigación original.
Conozca más sobre el Centro
Clínica
Una clínica pionera para alinear la atención entre las especialidades de trasplante y cáncer.
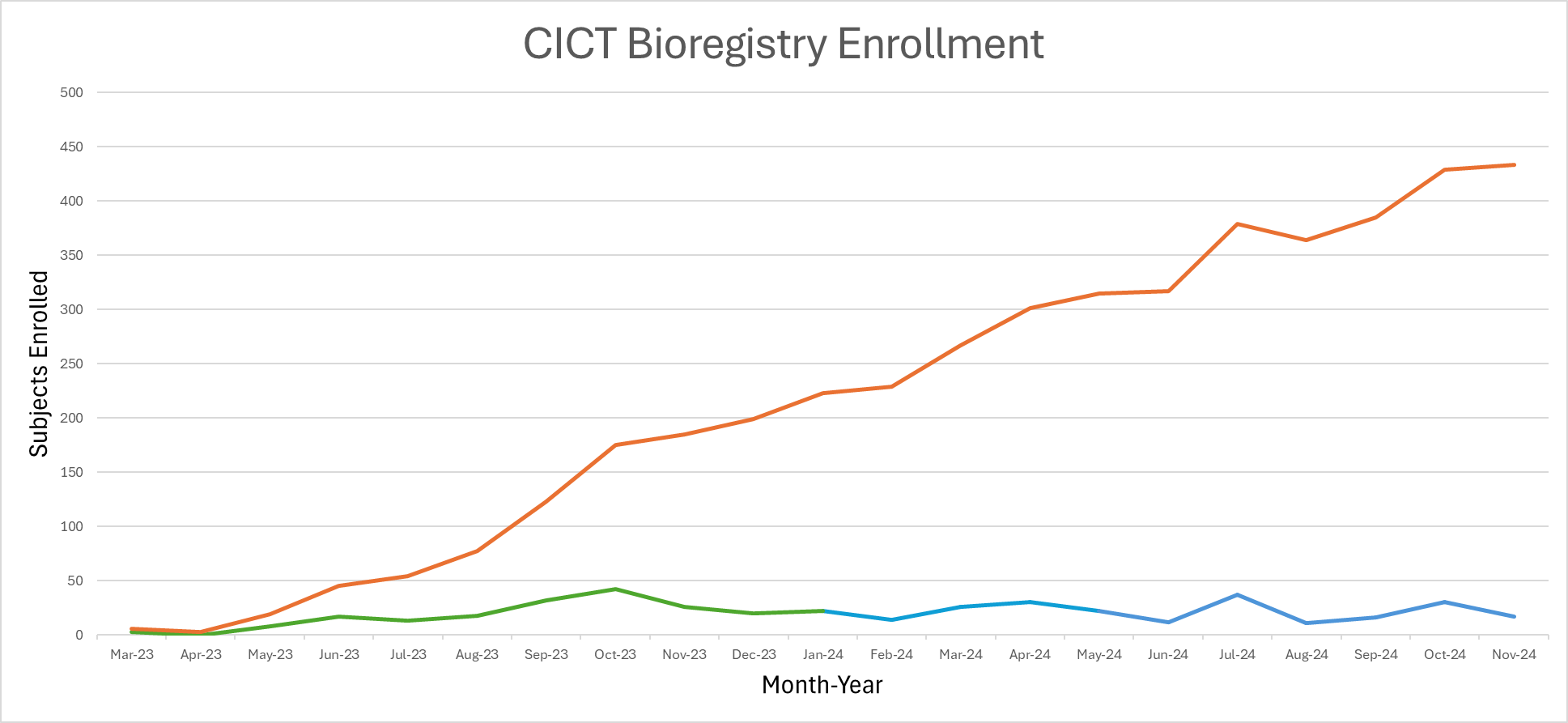
Biorregistro
Un registro para integrar los datos de pacientes de múltiples centros oncológicos y programas de trasplante de todo el mundo.
Investigación
Investigar los mecanismos inmunológicos y la epidemiología del cáncer con trasplante de órganos sólidos.
Publicaciones recientes
Real-world evidence regarding cancer, mortality, and graft failure risk with de novo belatacept use among kidney transplant recipients in the United States
Shyfuddin Ahmed…Christopher D. Blosser…Eric A. Engels
American Journal of Transplantation
Original Work
DOI: 10.1016/j.ajt.2025.03.004
We used multivariable Cox regression models to compare incidence of invasive cancer, cutaneous squamous cell carcinoma (cSCC), posttransplant lymphoproliferative disorder (PTLD), death, and graft failure/retransplantation (GF/RT) between belatacept and tacrolimus users. Overall, cancer incidence was 10.1 and 12.6 per 1000 person-years in belatacept and tacrolimus users, respectively. We did not find increased risk with belatacept for cancer overall (adjusted hazard ratio [HR] 0.83, 95% confidence interval [95%CI] 0.53-1.30), individual cancer types, or cSCC. Belatacept was associated with increased risk of death (adjusted HR 1.22, 95%CI 1.04-1.43) but lower risk of GF/RT more than four years after transplantation (0.54, 0.35-0.83). PTLD risk was increased among EBV-seropositive KTRs (adjusted HR 1.96, 95%CI 1.03-3.73). This study provides reassurance that belatacept does not increase cancer risk among KTRs, and there was a long-term protective association for GF/RT. However, we found evidence suggesting a potential increased risk of PTLD and death with belatacept use.
PTLD – It’s Best to Face This Growing Challenge at the Start
Christopher D. Blosser, Lianna J. Marks, Vikas R. Dharnidharka
American Journal of Transplantation
Editorial
DOI: 10.1016/j.ajt.2025.03.006
Post-transplant lymphoproliferative disorder (PTLD) is a leading cancer cause of death in kidney transplant recipients (KTRs). Understanding the risk factors that predispose KTRs to PTLD is a crucial step in developing strategies to mitigate this serious complication and improve patient outcomes.
Cure models, survival probabilities, and solid organ transplantation for patients with colorectal cancer
Eric A Engels… Christopher D Blosser…Ruth M Pfeiffer
American Journal of Transplantation
Original Work
DOI: 10.1016/j.ajt.2024.08.018
PMID: 39182612
A previous cancer diagnosis can preclude patients from consideration for solid organ transplantation. Statistical models may improve candidate selection. We fitted statistical cure models and estimated 5-year cancer-specific survival (5yCSS) for colorectal cancer patients in the United States using registry data. The median cure probability at cancer diagnosis for patients in the general population was 0.67.
Among 956 colorectal cancer patients who underwent solid organ transplantation, the median time since diagnosis was 6.3 years and the median 5yCSS at transplantation was 0.96. Patients with a 5yCSS below 0.90 had increased posttransplant cancer-specific mortality (hazard ratio 3.31, 95% CI 1.52-7.21). Compared with recently published guidelines, our models suggested shorter wait times for some groups of colorectal cancer patients (eg, stage IIA cancers) and longer wait times for others (stages IIB, IIIB, IIIC, IV). In conclusion, colorectal cancer patients undergoing solid organ transplantation had excellent prognoses, reflecting selection incorporating existing guidelines and clinical judgment. Nonetheless, 5yCSS probabilities estimated from cure models offer additional prognostic information for patients considered for transplantation and identify situations where current guidelines might be revised. We developed a web-based tool for clinicians to calculate 5yCSS probabilities for use in transplant evaluation for individual colorectal cancer patients